LBF20406CV18
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8034 |
| LipidMaps | LMFA03120015 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV18 |
| Clavulolactone I | |
|---|---|
| |
| Structural Information | |
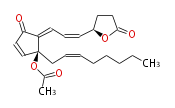
| 4R-{ (1-cis,3-trans) -3-[ 2S-acetoxy-2- [cis-2-octenyl] -5-oxo-3-cyclopentenylidene ] -1-propenyl}-4-butanolide | |
| |
| Formula | C22H28O5 |
| Exact Mass | 372.193674006 |
| Average Mass | 372.45472000000007 |
| SMILES | CC(=O)O[C@@](CC=CCCCCC)(C=2)C(C(C2)=O)=CC=C[C@H](O1)CCC(=O)1 |
| Physicochemical Information | |
| [ α ]D -7.8°(C 0.26, CHCl3) IwashimaMet al. | |
| Clavulolactones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iwashima_M et al. Iguchi_K et al. | |
| Clavulolactone I was converted from clavulone I. Iwashima_M et al. | |
| Spectral Information | |
| Mass Spectra | EIMS m/z 372 (M+). HREIMS m/z 372.1916 for C22}H_{28O5, calcd 372.1937. IwashimaMet al. |
| UV Spectra | λ EtOH max 231 nm(log ε 4.14),292 nm(log ε 4.20) IwashimaMet al. |
| IR Spectra | ν film max 1770,1732, 1704, 1643, and 1230cm-1 IwashimaMet al. |
| NMR Spectra | 1H-NMR(500MHz,CDCl3) δ ppm0.88(3H,t,J=6.9Hz),1.20-1.35(6H,m),1.94(2H,m),1.97-2.04(1H,m),2.04(3H,s),2.48-2.58(1H,m),2.60(1H,d,J=9.5Hz),2.62(1H,dd,J=2.5,9.5Hz),2.70(1H,dd,J=8.3,14.6Hz),2.93(1H,dd,J=6.9,14.6Hz),5.18(1H,ddd,J=6.9,8.3,10.8Hz),5.53(1H,q,J=7.3Hz),5.53-5.59(1H,m),6.01(1H,ddd,J=0.6,8.7,10.9Hz),6.45(1H,d,J=6.1Hz),6.67(1H,ddd,J=1.2,10.9,12.8Hz),7.01(1H,d,J=12.8Hz),7.48(1H,d,J=6.1Hz). IwashimaMet al. 13C-NMR(125MHz,CDCl3) δ ppm14.0,21.3,22.5,27.4,29.7,29.0,29.3,31.5,36.0,75.5,85.1,120.7,123.3,125.0,135.1,135.4,138.2,138.4,158.2,169.2,176.3,193.3. IwashimaMet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|