LBF20406HP09
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8089 |
| LipidMaps | LMFA03060048 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HP09 |
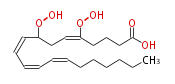
| 8,15-Dihydroperoxy-5,9,11,13-eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 8,15-Dihydroperoxy-5,9,11,13-eicosatetraenoic acid | |
| |
| Formula | C20H32O6 |
| Exact Mass | 368.219888756 |
| Average Mass | 368.46448 |
| SMILES | C(CCC(OO)C=CC=CC=CC(OO)CC=CCCCC(O)=O)CC |
| Physicochemical Information | |
| Autooxidation of arachidonic acid Yamagata_S et al.. In degradation products of 15-hydroperoxides of arachidonic acid in the presence of Fe(III)-ascorbic acid. Oxidation of arachidonic acid by singlet-oxygen Terao_J et al.. It is produced enzymatically from 15-HPETE in vivo(8,15-DHPETE). It is produced from 15-HPETE by soy bean lipoxygenase Bild_GS et al.. | |
| Spectral Information | |
| Mass Spectra | EI-MS(Me-ester; after reduction) Bild_GS et al.: m/e=350[M]; 332[M-H2O]; 301[332-OCH3]; 261[332-(CH2)4CH3]; 209[M-CH=CH(CH2)4COOCH3]; 191[209-H2O]; GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS) YamagataSet al. TeraoJet al.: m/e=487[M-CH3]; 431[M-(CH2)4CH3]; 359[M-(CH2)6COOCH3] |
| UV Spectra | UV(Me-ester) TeraoJet al. conjugated triene: 270nm and 281nm |
| IR Spectra | IR(Me-estre) TeraoJet al.OOH group: 3400cm-1, IR(after reduction) Bild_GS et al. trans unsaturation: 968cm-1, OH group: 1040, 3540cm-1 |
| NMR Spectra | 1H-NMR(Me-ester) TeraoJet al. OOH: 8.3ppm, 1H-NMR(after reduction) Bild_GS et al. C8, C15: 4.2ppm; OH: 6.3ppm |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|