LBF20406HP10
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8082 |
| LipidMaps | LMFA03060041 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HP10 |
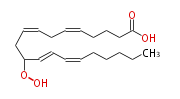
| 11-Hydroperoxy- 5,8,12,14 -eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 11-Hydroperoxy- 5,8,12,14 -eicosatetraenoic acid | |
| |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC=CC=CC(OO)CC=CCC=CCCCC(O)=O)CCC |
| Physicochemical Information | |
| Autooxidation of arachidonic acid Terao_J et al. Yamagata_S et al. Porter_NA et al.. Oxidation of arachidonic acid by singlet-oxygen Terao_J et al. Porter_NA et al.. Reaction products between arachidonic acid and lipoxygenase from living cells(11-HPETE). | |
| 11-HPETE generated by 11-lipoxygenase is enzymatically converted to bioactive compounds such as leukotriene and HETE Spector_AA et al.. | |
| Isomerization of hydroperoxides : Both 5- and 15-hydroperoxide were generated predominantly by autooxidation or singlet-oxygen mediated oxidation Terao_J et al. Yamagata_S et al. Peers_KE et al.. However, the proportion of each isomer generated become almost equal by supplementation of hydrogen donor such as tocopherol Porter_NA et al. Terao_J et al. Peers_KE et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(Me-ester; after reduction and TMS) TeraoJet al. TeraoJet al. RabinovitchHet al., GC-EI-MS(Me-ester; after reduction and TBDMS)(114), GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS) TeraoJet al. TeraoJet al. Porter_NA et al. Porter_NA et al. MayerBet al., GC-EI-MS(Me-ester; after reduction, hydrogenation and TBDMS) |
| UV Spectra | UV Porter_NA et al. conjugated diene: λ max=235nm, UV(Me-ester) TeraoJet al. conjugated diene: λ max=232.5nm, UV(Me-ester; after reduction) Porter_NA et al. conjugated trans, cis diene: λ max=236nm, conjugated trans, trans diene: λ max=232.5 |
| IR Spectra | IR Porter_NA et al.: conjugated trans, cis diene: 985, 950cm-1, OOH group: 3400cm-1, IR(Me-ester; after reduction) Porter_NA et al.conjugated trans, cis diene: 985, 950cm-1, conjugated trans, trans diene: 989cm-1 |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|