LBF18303HP04: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA8054 | |LipidBank=DFA8054 | ||
|LipidMaps=LMFA01040038 | |LipidMaps=LMFA01040038 | ||
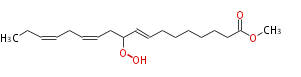
|SysName=Methyl-10- | |SysName=Methyl-10-hydroperoxy- (trans-8,cis-12,cis-15) -octadecatrienoic acid | ||
|Common Name=&&Methyl-10- | |Common Name=&&Methyl-10-hydroperoxy- (8E,12Z,15Z) -octadecatrienoic acid&& | ||
|Mass Spectra=EI-MS(Me-ester; after reduction and hydrogenation)[[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]]: m/e=201[O=CH(CH2)8C(=OH)OCH3]; 172[CH2(CH2)7C(=O)OCH3];169[O=CH(CH2)8C=O]; GC-EI-MS (Me-ester; after reduction, TMS)[[Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234}}]]: m/e=271[SMTO=CH-CH=CH(CH2)6COOCH3], GC-EI-MS(Me-ester;after reduction, hydrogenation) | |Mass Spectra=EI-MS(Me-ester; after reduction and hydrogenation)[[Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100|{{RelationTable/GetFirstAuthor|Reference:Chan_HWS:,J. Am. Oil Chem. Soc.,1977,54,100}}]]: m/e=201[O=CH(CH2)8C(=OH)OCH3]; 172[CH2(CH2)7C(=O)OCH3];169[O=CH(CH2)8C=O]; GC-EI-MS (Me-ester; after reduction, TMS)[[Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,J. Am. Oil Chem. Soc.,1977,54,234}}]]: m/e=271[SMTO=CH-CH=CH(CH2)6COOCH3], GC-EI-MS(Me-ester;after reduction, hydrogenation) | ||
|Source=Oxidation of linoleate by singlet oxygen[[Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197}}]] | |Source=Oxidation of linoleate by singlet oxygen<!--8022--><!--8023-->[[Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1980,19,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1983,22,1}}]][[Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:,Prog. Lipid Res.,1984,23,197}}]]<!--8027--><!--8030-->. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity=Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously.[[Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485|{{RelationTable/GetFirstAuthor|Reference:Logani_MK:Davies_RE:,Lipids,1980,15,485}}]]<!--8044--><!--8045-->[[Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365|{{RelationTable/GetFirstAuthor|Reference:Sevanian_A:Hochstein_P:,Annu. Rev. Nutr.,1985,5,365}}]][[Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21}}]]<!--8048--><!--8049-->. It reacts with DNA in the presence of Fe ions and ascorbic acid[[Reference:Fujimoto_K:Neff_WE:Frankel_EN:,Biochim. Biophys. Acta,1984,795,100|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:Neff_WE:Frankel_EN:,Biochim. Biophys. Acta,1984,795,100}}]]. | |||
|Note=Isomerization of hydroperoxides: Positional isomers at 9, 10, 12, 13, 15, and 16 and their geometric isomers of cis, cis, trans- or cis, trans, trans are formed. The proportion of 9-, 16- isomers is higher than that of 12-, 13- isomers. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 18:51, 29 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8054 |
| LipidMaps | LMFA01040038 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18303HP04 |
| Methyl-10-hydroperoxy- (8E,12Z,15Z) -octadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| Methyl-10-hydroperoxy- (trans-8,cis-12,cis-15) -octadecatrienoic acid | |
| |
| Formula | C19H32O4 |
| Exact Mass | 324.23005951199997 |
| Average Mass | 324.45498 |
| SMILES | CCC=CCC=CCC(OO)C=CCCCCCCC(=O)OC |
| Physicochemical Information | |
| Oxidation of linoleate by singlet oxygen Frankel_EN Frankel_EN Frankel_EN . | |
| Pysiological damages are induced by these hydroperoxides which are incorporated into bodies or synthesized endogenously. Logani_MK et al. Sevanian_A et al. Fujimoto_K . It reacts with DNA in the presence of Fe ions and ascorbic acid Fujimoto_K et al.. | |
| Isomerization of hydroperoxides: Positional isomers at 9, 10, 12, 13, 15, and 16 and their geometric isomers of cis, cis, trans- or cis, trans, trans are formed. The proportion of 9-, 16- isomers is higher than that of 12-, 13- isomers. | |
| Spectral Information | |
| Mass Spectra | EI-MS(Me-ester; after reduction and hydrogenation) Chan_HWS : m/e=201[O=CH(CH2)8C(=OH)OCH3]; 172[CH2(CH2)7C(=O)OCH3];169[O=CH(CH2)8C=O]; GC-EI-MS (Me-ester; after reduction, TMS) TeraoJet al.: m/e=271[SMTO=CH-CH=CH(CH2)6COOCH3], GC-EI-MS(Me-ester;after reduction, hydrogenation) |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|