LBF20406HO18: Difference between revisions
No edit summary |
No edit summary |
||
| (15 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=DFA8136 | |LipidBank=DFA8136 | ||
|LipidMaps=LMFA03060008 | |LipidMaps=LMFA03060008 | ||
|SysName=12R- | |SysName=12R-Hydroxy- (cis-5,cis-8,trans-10,cis-14) -eicosatetraenoic acid | ||
|Common Name=&&12R- | |Common Name=&&12R-Hydroxy- (5Z,8Z,10E,14Z) -eicosatetraenoic acid&& | ||
|UV Spectra= | |UV Spectra= lambda max: 237nm epsilon : 27,000 | ||
|Source=12(R)-HETE is produced by the action of 12(R)-lipoxygenase on arachidonic acid [[Reference:Hawkins_DJ:Brash_AR:,J. Biol. Chem.,1987,262,7629|{{RelationTable/GetFirstAuthor|Reference:Hawkins_DJ:Brash_AR:,J. Biol. Chem.,1987,262,7629}}]]. | |||
|Chemical Synthesis= | |||
|Metabolism=12(R)-HETE is the predominant stereoisomer produced by psoriatic skin scales and rat liver microsomal cytochrome P-450 [[Reference:Woollard_PM:,Biochem. Biophys. Res. Commun.,1986,136,169|{{RelationTable/GetFirstAuthor|Reference:Woollard_PM:,Biochem. Biophys. Res. Commun.,1986,136,169}}]][[Reference:Capdevila_J:Yadagiri_P:Manna_S:Falck_JR:,Biochem. Biophys. Res. Commun.,1986,141,1007|{{RelationTable/GetFirstAuthor|Reference:Capdevila_J:Yadagiri_P:Manna_S:Falck_JR:,Biochem. Biophys. Res. Commun.,1986,141,1007}}]]. | |||
|Symbol=12(R)-HETE | |||
|Biological Activity=12(R)-HETE is the primary lipoxygenase product in invertebrates such as the sea urchin where it plays a role in reproduction physiology [[Reference:Hawkins_DJ:Brash_AR:,J. Biol. Chem.,1987,262,7629|{{RelationTable/GetFirstAuthor|Reference:Hawkins_DJ:Brash_AR:,J. Biol. Chem.,1987,262,7629}}]].1 12(R)-HETE has been isolated from several tissues in mammals, but it is controversial whether it is derived from cytochrome P450 or 12(R)-lipoxygenase metabolism, in some cases. 12(R)-HETE is a potent chemotactic factor exhibiting dose-dependent neutrophil chemotaxis with significant responses observed at doses as low as 10 ^{-11} M [[Reference:Masferrer_JL:Rimarachin_JA:Gerritsen_ME:Falck_JR:Yadagiri_P:Dunn_MW:Laniado-Schwartzman_M:,Exp. Eye Res.,1991,52,417|{{RelationTable/GetFirstAuthor|Reference:Masferrer_JL:Rimarachin_JA:Gerritsen_ME:Falck_JR:Yadagiri_P:Dunn_MW:Laniado-Schwartzman_M:,Exp. Eye Res.,1991,52,417}}]]. It produces neovascularization in rabbit corneas at 0.5 μg. | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 16:27, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8136 |
| LipidMaps | LMFA03060008 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HO18 |
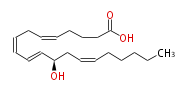
| 12R-Hydroxy- (5Z,8Z,10E,14Z) -eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 12R-Hydroxy- (cis-5,cis-8,trans-10,cis-14) -eicosatetraenoic acid | |
| |
| 12(R)-HETE | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC=CCC(C=CC=CCC=CCCCC(O)=O)O)CCC |
| Physicochemical Information | |
| 12(R)-HETE is produced by the action of 12(R)-lipoxygenase on arachidonic acid Hawkins_DJ et al.. | |
| 12(R)-HETE is the predominant stereoisomer produced by psoriatic skin scales and rat liver microsomal cytochrome P-450 Woollard_PM Capdevila_J et al.. | |
| 12(R)-HETE is the primary lipoxygenase product in invertebrates such as the sea urchin where it plays a role in reproduction physiology Hawkins_DJ et al..1 12(R)-HETE has been isolated from several tissues in mammals, but it is controversial whether it is derived from cytochrome P450 or 12(R)-lipoxygenase metabolism, in some cases. 12(R)-HETE is a potent chemotactic factor exhibiting dose-dependent neutrophil chemotaxis with significant responses observed at doses as low as 10 ^{-11} M Masferrer_JL et al.. It produces neovascularization in rabbit corneas at 0.5 μg. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 237nm ε : 27,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|