Sphingolipid
Upper classes:
LB
Class Overview
Three Sphingolipid categories (LBS) exist in this database.
|
このデータベースではスフィンゴ脂質を以下に分けています。
|
Ceramide
|
| |
OH |
| (C14-chain) | = | / | \ | / | ー | OH |
| (fatty acid) ー |
| |
NH |
セラミド
|
Glycosphingolipid
|
| |
OH |
| (C14-chain) | = | / | \ | / | ー | O-(sugar) |
| (fatty acid) ー |
| |
NH |
スフィンゴ糖脂質
|
Phosphosphingolipid
|
| |
OH |
| (C14-chain) | = | / | \ | / | ー | O-(phosphate) |
| (fatty acid) ー |
| |
NH |
スフィンゴリン脂質
|
|
|
|
|
Sphingolipid
Notation
| Symbol Abbreviations (e.g. Glc = glucose)
|
| Backbones
|
| Cer |
 |
ceramide |
セラミド
|
| Sph |
 |
sphingosine |
スフィンゴシン
|
| Sugars
|
| Glc |
 |
glucose |
グルコース
|
| Gal |
 |
galactose |
ガラクトース
|
| GlcNAc |
 |
N-acetyl-glucosamine |
N-アセチルグルコサミン
|
| GalNAc |
 |
N-acetyl-galactosamine |
N-アセチルガラクトサミン
|
| GlcN |
 |
glucosamine |
グルコサミン
|
| GalN |
 |
galactosamine |
ガラクトサミン
|
| GlcA |
 |
glucuronic acid |
グルクロン酸
|
| GalA |
 |
galacturonic acid |
ガラクツロン酸
|
| Man |
 |
mannose |
マンノース
|
| Rha |
 |
rhamnose (= 6-deoxy-mannose) |
ラムノース (6-デオキシマンノース)
|
| Ins |
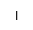 |
inositol |
イノシトール
|
| Fuc |
 |
fucose (= 6-deoxy-galactose) |
フコース (6-デオキシガラクトース)
|
| Ara |
 |
arabinose |
アラビノース
|
| Xyl |
 |
xylose |
キシロース
|
| NeuNH |
 |
(deacetyl) neuraminic acid
(pyruvate + mannosamine) |
ノイラミン酸
(ピルビン酸+マンノサミン)
|
| NeuAc |
 |
N-acetyl-neuraminic acid |
N-アセチルノイラミン酸
|
| NeuGc |
 |
N-glycolyl-neuraminic acid |
N-グリコリルノイラミン酸
|
| Kdn |
 |
2-keto-3-deoxy-D-glycero-
D-galacto-nononic acid |
デアミノノイラミン酸
|
| Modifiers
|
| AEPn |
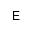 |
aminoethylphosphonic acid, or
(2-aminoethyl)hydroxyphosphoryl- |
アミノエチルホスホン酸(C-P結合)
|
| MAEPn |
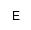 |
N-methyl aminoethylphosphonic acid, or
(N-methyl-2-aminoethyl) hydroxyphosphoryl- |
AEPnのN-メチル体(C-P結合)
|
| EtnP |
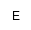 |
phosphoethanolamine (PEA), or
2-aminoethanolphospho- |
ホスホエタノールアミン
|
| PC |
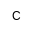 |
Phosphocholine |
ホスホコリン
|
| Butenyl |
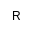 |
Butenyl |
ブテニル基
|
| Me |
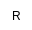 |
Methyl |
メチル基
|
| Et |
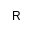 |
Ethyl |
エチル基
|
Page/ID-Code Design
Major series of phosphosphingolipids are determined by molecules bound to their phosphates, and the series of glycosphingolipids are determined by oligosaccharides bound to their ceramides. The IUPAC series of glycosphingolipids (1977) were based on the order and bonding patterns of four sugars rooting at the long-chain base. Our series, however, are based on three sugars. Two original series, Echino and Schisto, are also added.
|
スフィンゴリン脂質はリン酸に結合する分子や糖の種類、スフィンゴ糖脂質はセラミドに結合する糖鎖により系列を作成しています。1977年にIUPACが提唱したスフィンゴ糖脂質の系列は、長鎖塩基に結合する四糖の並びと結合を基準にしています。ここではより広い糖鎖の系列を検索できるよう、三糖を基準に分類しています。また新たにEchinoとSchistoの系列を加えています。
|
| L
| B
| S
| G/P
| s1
| s2
| n1
| n2
| y
| c1
| c2
|
|
|
| G/P |
G (glyco) |
P (phospho)
|
| s1, s2
|
structure code as explained below
|
| n1, n2
|
number of sugars (2 digits)
|
| y
|
number of sialic acids (1 digit)
|
| c1, c2
|
serial numbers
|
|
First (s1) Digit for Glycosphingolipid
| First sugar |
Code |
Root sequence
|
Major Series
|
| Cerebroside
|
For GalCer and GlcCer, visit this page.
|
starting with
Galactose
|
()
|
Gal-Cer
|
|
|
Galα1-4/6Galβ1-Cer
|
|
|
Galβ1-4Glcβ1-3Galβ1-Cer
|
|
starting with
Glucose
|
()
|
GalNAcβ-Galβ-Glcβ-Cer
|
|
|
GalNAcβ1-4Galβ1-4Glcβ1-Cer
|
| [1]
|
GalNAcβ1-3Galβ1-4Glcβ1-Cer
|
|
|
GlcNAcβ1-3(GalNAcβ1-4)Galβ1-4Glcβ1-Cer
|
|
|
GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-Cer
|
|
|
GalNAcβ1-4(NeuAcα2-8NeuAcα2-3)Galβ1-4Glcβ1-Cer
|
|
|
GalNAcβ1-4(NeuAcα2-8NeuAcα2-8NeuAcα2-3)Galβ1-4Glcβ1-Cer
|
|
|
...(NeuAcα2-6)GalNAcβ1-4Galβ1-4Glcβ1-Cer
|
|
|
...(NeuAcα2-8NeuAcα2-6)GalNAcβ1-4Galβ1-4Glcβ1-Cer
|
|
()
|
GlcNAcβ-Galβ-Glcβ-Cer
|
|
|
Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ1-Cer
|
| [2]
|
Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-Cer
|
|
()
|
Galα-Galβ-Glcβ-Cer
|
|
|
Galα1-4Galβ1-4Glcβ1-Cer
|
| [1]
|
Galα1-3Galβ1-4Glcβ1-Cer
|
|
()
|
Gal-Glc-Cer
(Other than G2∼G4)
|
|
()
|
Man-Glcβ-Cer
|
|
|
GlcNAcβ1-3Manβ1-4Glcβ1-Cer
|
|
|
Manα1-3Manβ1-4Glcβ1-Cer
|
|
()
|
NeuAc/NeuGc-Glcβ-Cer
|
|
|
NeuGc or NeuAcα2-6Glcβ1-Cer
|
|
()
|
GalNAc-Glc-Cer
|
|
starting with
Other Sugars
|
|
Symbols follow the Sugar-digit table.
|
- ↑ 1.0 1.1 In IUPAC, "iso" refers to a change from position 4 to position 3.
- ↑ In IUPAC, "neo" refers to a change from position 3 to position 4.
- ↑ Makaaru CK, Damian RT, Smith DF, Cummings RD. "The human blood fluke Schistosoma mansoni synthesizes a novel type of glycosphingolipid" J Biol Chem 1992 267:2251-2257 PMID 1733932
First (s1) Digit for Phosphosphingolipid
| First sugar |
Code |
Root sequence
|
Major Series
|
| No Sugar Attached
|
P1 (LBSP)
|
R-P-Cer
|
Sphingomyelin and Ceramide phosphoetanolamine
(Visit this page.)
|
starting with
Inositol
|
(29 items)
|
GlcNα/β1−2/6Ins1-P-Cer
|
|
(10 items)
|
GlcAα/β1−2/6Ins1-P-Cer
|
|
(52 items)
|
Manα/β1−2/6Ins1-P-Cer
|
|
(21 items)
|
Gal, Glc, and Others
|
X-Ins1-P-Cer
|
Second (s2) Digit
Click to see the full list. For abbreviations, see #Notation.
| Code |
Structure
|
| A
|
Glc 
|
| B
|
Gal 
|
| C
|
GlcNAc 
|
| D
|
GalNAc 
|
| E
|
GlcN 
|
| F
|
GalN 
|
| G
|
GlcA 
|
| H
|
GalA 
|
| I
|
not used
|
|
| Code |
Structure
|
| J
|

|
| K
|

|
| L
|
|
| M
|
Fucose (, , ) 
|
| N
|

|
| O
|

|
| P
|

|
| Q
|

|
| R
|

|
|
| Code |
Structure
|
| S
|
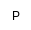
|
| T
|
, , 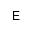
|
| U
|
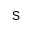
|
| V
|
acyl/alkyl/alkenyl chain 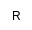
|
Biosynthesis
Sphingosine is synthesized from decarboxylating condensation of palmitoyl CoA with serine on the ER membrane. It is a 18-carbon amino alcohol (16 carbons from palmitic acid and 2 from serine) with one unsaturated bond (trans-form) at the C-4 position (2-amino-4-octadecene-1,3-diol). (more details)
|
スフィンゴシンはパルミトイルCoAがアミノ酸セリンと脱炭酸縮合して生成します。炭素を18個含むアミノアルコール(16個がパルミチン酸、2個がセリン由来)でトランス型の不飽和結合をC-4に1つ含みます。(詳細はこちら)
|
History of Sphingolipids
Sphingolipids were first isolated from brain by J.L.W. Thudichum (1829-1901) as lipids of unknown function and structure.[1] The basic structure is a long-chain base (long-chain amino-alcohol) whose amino-group is acylated with a long-chain fatty acid. This hydrophobic structure is called ceramides.
Sphingolipid consists of phospho-sphingolipid and glyco-sphingolipid, whose ceramide structure is bonded with phosphate and (oligo)saccharides, respectively. Phosphosphingolipid includes sphingomyelin, and glycosphingolipid, cerebrosides and gangliosides. Both are abundant in neural tissues.[2]
|
スフィンゴ脂質は J.L.W. Thudichum (1829-1901) により機能や構造未知の脂質として脳から見いだされました。基本骨格は長鎖塩基 (長鎖アミノアルコール) のアミノ基が長鎖脂肪酸とアミド結合したもので、セラミドと呼ばれます。
スフィンゴ脂質はセラミドにリン酸が結合したスフィンゴリン脂質と糖が結合したスフィンゴ糖脂質に分けられます。スフィンゴリン脂質にはスフィンゴミエリンが、スフィンゴ糖脂質にはセレブロシドやガングリオシドなどが含まれ、いずれも神経系に多く存在します。
|
- ↑ Thudichum J.L.W.?"A Treatise on the Chemical Constitution of the Balliere" Tindall and Cox, London,?1884
- ↑ Gault CR,?Obeid LM,?Hannun YA "An overview of sphingolipid synthesis to breakdown" Adv Exp Med Biol.?2010;688:1-23. PMID 20919643
|
Subcategories
This category has the following 4 subcategories, out of 4 total.