LBF20406CV14
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8010 |
| LipidMaps | LMFA03120010 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV14 |
| Chlorovulone III | |
|---|---|
| |
| Structural Information | |
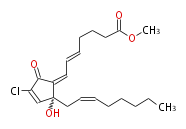
| Methyl- (trans-5,cis-7) -7- [ 4R-chloro-2-hydroxy-2- (cis-2-octenyl) -5-oxo-3-cyclopentenylidene ] -5-heptenoic acid | |
| |
| Formula | C21H29ClO4 |
| Exact Mass | 380.175437126 |
| Average Mass | 380.90525999999994 |
| SMILES | C(C(=O)1)(Cl)=C[C@](CC=CCCCCC)(C(=CC=CCCCC(OC)=O)1)O |
| Physicochemical Information | |
| [ α ]D +27.3°(C 0.033, CHCl3) IguchiKet al. | |
| Chlorovulones are soluble in MeOH, EtOH, CHCl3, or hexane. | |
| Chlorovulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iguchi_K et al. Nagaoka_H et al. | |
| Photoisomerization of chlorovulone I (fluoresent lamp, benzene, 40 hr) gave a mixture of chlorovulones I, II and III. Iguchi_K et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max(EtOH) 238 nm( ε 13200),315 nm( ε 11900) IguchiKet al. |
| IR Spectra | ν max(CHCl3)1725,1700, and 1630cm-1(Yamada Yasuji) |
| NMR Spectra | 1H-NMR(400MHz,CDCl3) δ ppm0.88(3H,t,J=7.1Hz),1.30(6H,m),1.81(2H,quint.,J=7.5Hz),2.00(2H,brq,J=6.9Hz),2.30(2H,brq,J=7.5Hz),2.35(2H,t,J=7.5Hz),2.55(1H,brdd,J=7.6,14.4Hz),2.67(1H,brdd,J=8.3,14.4Hz),3.68(3H,s),5.29(1H,ttd,J=1.7,7.6,10.9Hz),5.38(1H,brtd,J=7.4,10.9Hz),6.19(1H,td,J=7.1,15.7Hz),6.68(1H,d,J=11.4Hz),7.15(1H,s),7.56(1H,tdd,J=1.4,11.4,15.7Hz). IguchiKet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|